
What’s something that looks brilliant and beautiful but you can never touch? Meet Mercury – the element marked Hg on the periodic table. The symbol Hg comes from the name hydrargyrum, which means “water-silver.” A shiny element whose properties make it extremely interesting to learn about. Named after the Roman god Mercury, who served as a messenger to all the other gods, Mercury is often called ‘quicksilver’ in reference to its high molecular mobility. Speed and mobility are characteristics of the god and the element alike!
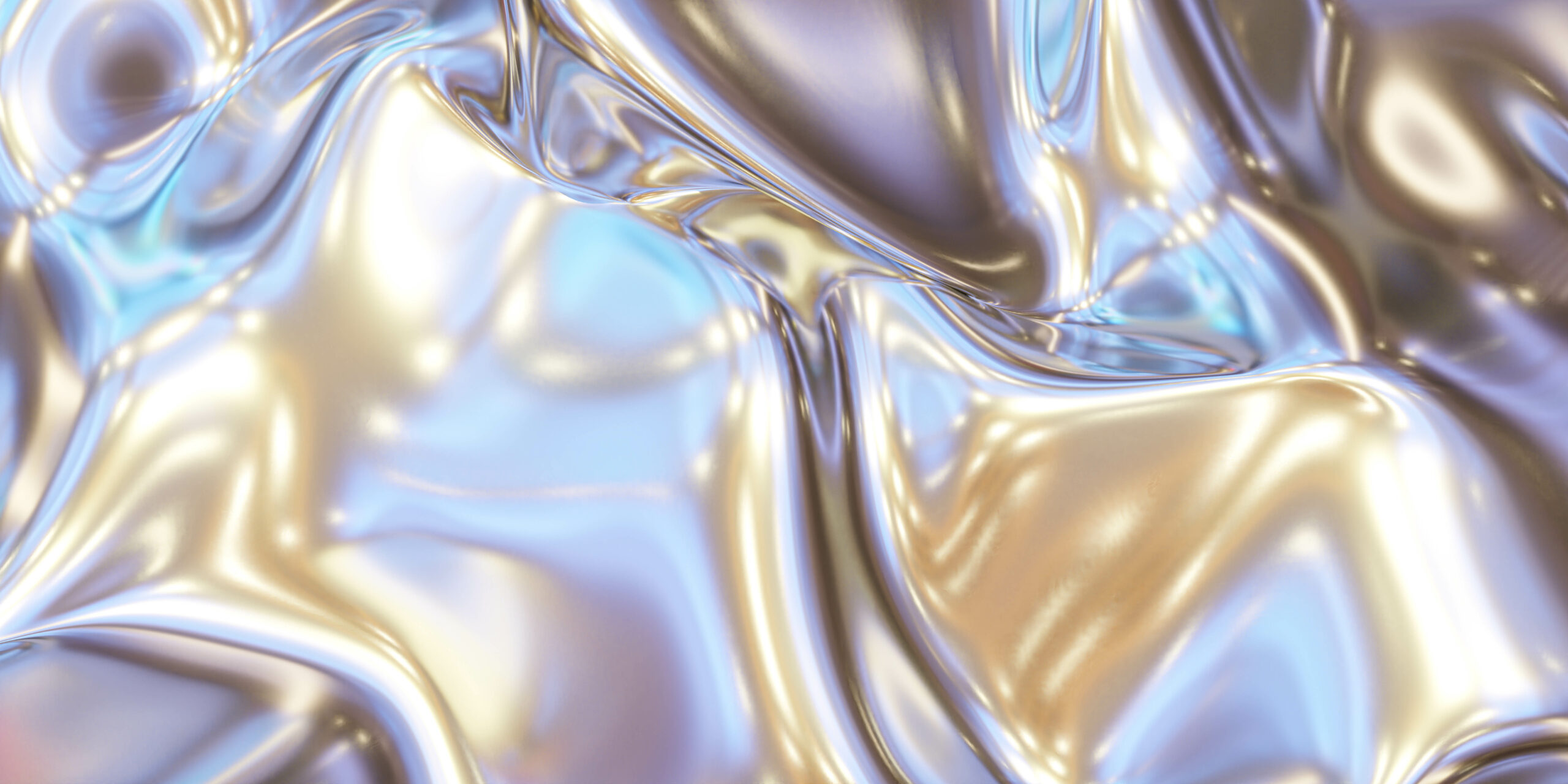
Here’s the first thing that makes Mercury so exciting. Mercury is a chemical element and the only common metal that is liquid at ordinary temperatures. Mercury at room temperature is a silvery-white liquid metal that looks like it’s part of a futuristic world! Mercury is unique because it’s classified as a metal and comes in both liquid and solid forms depending on the temperature.
Mercury has been known for thousands of years, with people learning to make the metal from the important ore called Cinnabar. Cinnabar released mercury as a vapour when heated, after which the vapour was cooled down and captured as the silvery liquid we know Mercury as.

Mercury is a naturally-occurring chemical element found in rocks in the earth’s crust, including coal deposits. On the periodic table, it is identified as “Hg” with the atomic number 80. It exists in several forms, including elemental (metallic) mercury, inorganic mercury compounds, and other organic compounds like Methylmercury. As a chemical element, mercury cannot be created or destroyed. The same amount of Mercury has existed on the planet since the earth was formed. However, we cycle the Mercury in the environment as part of both natural and human activities.

Mercury can be frozen and changed into a solid at a temperature of –38.85°C. It is liquid at room temperature and can be transformed into a gas when boiled at 365.6°C. The density of mercury is 13.59 grams per cubic centimetre. It has two key physical properties – very high surface tension and being a very good conductor of electricity.
But before we go further, we must remember a key detail; Mercury is both volatile and poisonous! The element is toxic and harmful to the human body, causing mercury poisoning when our bodies are exposed to too much of it. Mercury poisoning can affect anyone who comes into contact with or consumes mercury, with the most common cause being the consumption of large amounts of fish with high mercury content or inhaling Mercury vapour. Mercury is known as an element with hazardous properties for the environment and workplace and is corrosive to many materials.

But let’s not get too caught up on the dark side, Mercury has found many new applications in electrical devices and electrochemistry! Elemental mercury can be found in thermometers, electricals, and dental fillings. Mercury happens to have several uses because it makes for a great conductor! This means that the compound allows electricity and heat to flow through it very well, making the usage of Mercury in thermometers, street lights and fluorescent light bulbs quite common.
Mercury is a very rare element in the Earth’s crust. It accounts for only about 0.08 parts per million (ppm) and is found primarily in the mineral cinnabar or mercuric sulfide, which is the source of the red pigment called vermilion.

In 2021, an estimated 2,300 metric tons of mercury was produced worldwide, a slight decrease from the 2,490 metric tons produced in the previous year. The major mercury-producing countries in the world are China, Kyrgyzstan, Mexico, Peru, Russia, Slovenia, Spain, and Ukraine, and have most of the world’s estimated 600,000 tons of mercury resources.
Did you find this article interesting? Let us know if you want to read more about elements like Mercury in the comments below!
Did you find this article interesting? Let us know if you want to read more about elements like Mercury in the comments below!
If you liked reading this, you can read more on Chemistry below:
Explore the wonders of Chemistry in everyday life